Ncert Cbse Solutions of Science (Chemistry)
Class X, CARBON AND ITS
COMPOUNDS
Answers of NCERT Science In-text Questions
Q.1: What would be the electron dot structure of carbon dioxide
which has the formula CO2 ?
Answer:
The electron dot structure of Carbon dioxide (CO2) is given below:
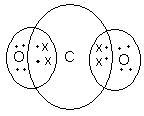
Q.2: What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur ?
Answer:
The 8 atoms of sulphur molecule (S8) are joined together in the form
of a ring as shown below:
Q.3: How many structural isomers can you draw for pentane ?
Q.3: How many structural isomers can you draw for pentane ?
Answer:
There are three structural isomers of pentane as given below:
Q.4: What are the two properties of carbon which lead to the huge number of carbon compound we see around us ?
Q.4: What are the two properties of carbon which lead to the huge number of carbon compound we see around us ?
Answer:
The two properties of carbon are – (a)
Catenation and (b) Tetra Covalency or
Tetravalency of carbon atom.
Catenation
is the unique property of carbon due to which carbon atoms can link among
themselves to form a straight, branched or close chain. Due to tetravalency,
the carbon atoms can form single, double or triple covalent bond. This is why carbon
leads to form a huge number of carbon compounds.
Q.5: What will be the formula and electron dot structure of
cyclopentane ?
Answer:
The formula of cyclopeptane is C5H10.

Q.6: Draw the structure for following compounds: (a) Ethanoic acid (b) Bromopentane (c) Butanone (d) Hexanal.

Q.6: Draw the structure for following compounds: (a) Ethanoic acid (b) Bromopentane (c) Butanone (d) Hexanal.
Answer:
(i) Bromo-ethane (ii) Methanal (iii) Hex 1-yne.
Q.8: Why is the conversion of ethanol to ethanoic acid an
oxidation reaction ?
Here, in this reaction there is a decrease in the number of hydrogen along with increase in number of oxygen atoms in compound. Therefore, it is an oxidation reaction.
Q.9: A mixture of oxygen and ethyne is burnt for welding. Can
you tell why a mixture of ethyne and air is not taken ?
Answer:
Air contains nitrogen and other inactive gaseous contents which resist the
adequate supply of oxygen for burning of ethyne. Ethyne is an unsaturated
hydrocarbon. If we use a mixture of ethyne and air then incomplete combustion
of ethyne takes place with a sooty flame and also high temperature required for
welding is not achieved. But if it is burnt with oxygen it produces clan flame
with very high temperature due to complete combustion.
Therefore,
air is taken for burning of ethyne for welding.
Q.10: How can you distinguish experimentally between an alcohol
and a carboxylic acid ?
Answer: We can distinguish experimentally between an
alcohol and a carboxylic acid by using laboratory reagent Na2CO3
solution as follows:
(i)
When Na2CO3 is added to the test tube containing CH3COOH,
CO2 gas evolves which turn the lime water milky.
(ii)
When Na2CO3 is added to the test tube containing CH3COOH,
no gas is evolved.
Q.11: Would you be able to check if water is hard by using a
detergent ?
Answer:
No, it is impossible because detergent is effective in both hard water and soft
water.
Q.12: People use a variety of methods to wash clothes. Usually
after adding the soap, they beat the clothes on a stone, or beat it with a
paddle, scrub with a brush or the mixture is agitated in a washing machine. Why
is agitation necessary to get clean clothes ?
Answer:
A soap molecule has two parts:
(i)
Hydrophobic part i.e. the hydrocarbon tail which is insoluble in water and
repelled by water.
(ii)
Hydrophilic part i.e. negatively charged end which is soluble in water.
With
the help of these parts, soap can attach grease and dirt particles and form
spherical clusters known as “micelle”. Due to ion-ion repulsion these micelles
remain suspended as a colloid in the water. In order to remove these micelles
containing the dirt, it is necessary to scrub or agitate the clothes.
Q.13: Why is detergent a better cleansing agent than soap ?
Answer:
Detergent a better cleansing agent than soap because detergent acts better even
in hard water.
Q.14: Which causes water pollution, detergent or soap ? ?
Answer:
Detergent causes water pollution as detergents are non-biodegradable.
Q.15: Why does not soap form lather with hard water ?
Answer:
Soap reacts with hard water to form scum and acts to remove the hardness of
water. So, lather is not formed by soap with hard water.
CBSE Class 10, CARBON AND ITS COMPOUNDS – Further study
- Carbon and its Compounds - NCERT Solutions of Exercise Questions | Class 10 CBSE Science (Chemistry)
- Chemistry Class X, NCERT (CBSE) Science | Carbon and its Compounds - MCQs (Part - 1)
- CBSE Class 10 Carbon and its Compounds | Ncert Cbse Science (Chemistry) | CCE type Hot Questions - Answers







No comments:
Write comments